The Art of Protein Crystallization
Enzymes catalyze many different chemical reactions in a cell and their three dimensional structure is the key to their function. As structure and function are linked, protein structures become more and more important in biology, biochemistry and medicine.
Why are protein structures important in the context of CEPLAS?
The climate change and declining area of arable land make it more and more difficult to feed the fast growing world population. Therefore, we need crops that use nutrients and light more efficient and can cope with drought much better than today’s crops. Plants, which have evolved towards C4 photosynthesis can do so. If we were are able to push our crops towards C4 photosynthesis, we might be able to achieve this aim. But before we can think of that, we have to understand the molecular differences between C3 and C4 photosynthesis in detail and therefore, we have to look at enzymes involved in C4 photosynthesis and learn more about their structure. In my project I want to solve the structure of a protein kinase involved in regulation of C4 photosynthesis.
However, solving the structure of a protein is not an easy task, as I can tell you!
To determine the structure of the regulatory kinase, I’m using X-ray diffraction analysis. First of all, I have to purify the protein and crystallize it. After that, the obtained protein crystal is exposed to an intense X-ray beam. The beam is diffracted by the crystal and from the diffraction pattern obtained, the protein structure can be calculated.
But initially, a protein crystal is required; and obtaining protein crystals can be hard work. Since each protein is different, no general protocol can be given for protein crystallization. Instead, crystallization conditions must be established in experiments for each protein. Multiple conditions must be screened using high throughput methods, because there are many parameters that affect protein crystallization, for example: pH, buffer, salts, additives, precipitants, temperature, protein concentration, and incubation time. Once a crystal is obtained, its diffraction properties must be analyzed by exposing it to an X-ray beam. The better the diffraction, the better the structure can be calculated.
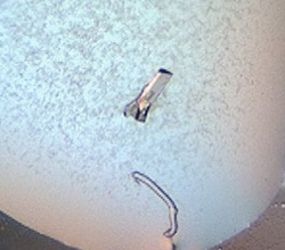
To start crystallization, my first job is to purify the protein kinase and concentrate it up to approximate 10 mg/mL. It sounds simple – but it’s not an easy task, if the protein tends to be insoluble. Next, the crystallization screens have to be set up. Commercially available crystallization screening kits, pipetting robots and automatic imaging systems of Heinrich-Heine University’s “X-ray Facility and Crystal Farm” help me to set up and screen a huge amount of different conditions within a short time. But so far, all crystals turned out to be salt instead of protein.
Fig 1: Two different kind of salt crystals obtained in my screens.
But how can I distinguish between salt and proteins in a fast way?
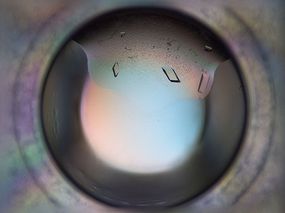
Well, (most) proteins contain the amino acid tryptophan, and tryptophan shows fluorescence under UV light. In contrast, salt shows no fluorescence. To give you an example of fluorescence of protein crystals, here is an image of L-ectoine synthase crystals under normal light and UV light:
Fig 2: Protein crystals under normal light and UV light (thanks to Stefanie Kobus!).
So, after working one year in the field of structural biology, I can tell you, that protein crystallization is a challenge. But there is also a big motivation for me: to contribute with protein crystals and hopefully new protein structures to new insights into C4 photosynthesis. A small step towards the aim to feed a growing world population.
Contribution by Johannes Schwabroh, Institute of Biochemical Plant Physiology, HHU
Planter’s Punch
Under the heading Planter’s Punch we present each month one special aspect of the CEPLAS research programme. All contributions are prepared by our young researchers.